Y-LAB: PRODUCTION AND LABORATORY DATA INTEGRITY DIGITAL SOLUTION
Connect. Analyze. Register.
Limitless!
Y-Lab is a LES (Laboratory Execution System) that aims to eliminate paper, digitizing the quality control laboratory, monitoring its performance and recording analysis data in real time.
Designed to integrate even poor (obsolete/dated) devices, it allows you to digitize the Quality Control department without replacing the existing equipments. It prevents early readings of analytical data and offers a solid tool, compliant with GMP, ensuring Data Integrity and Data Governance
Multipurpose devices integration (file transfer, start/stop logic, real-time process data)
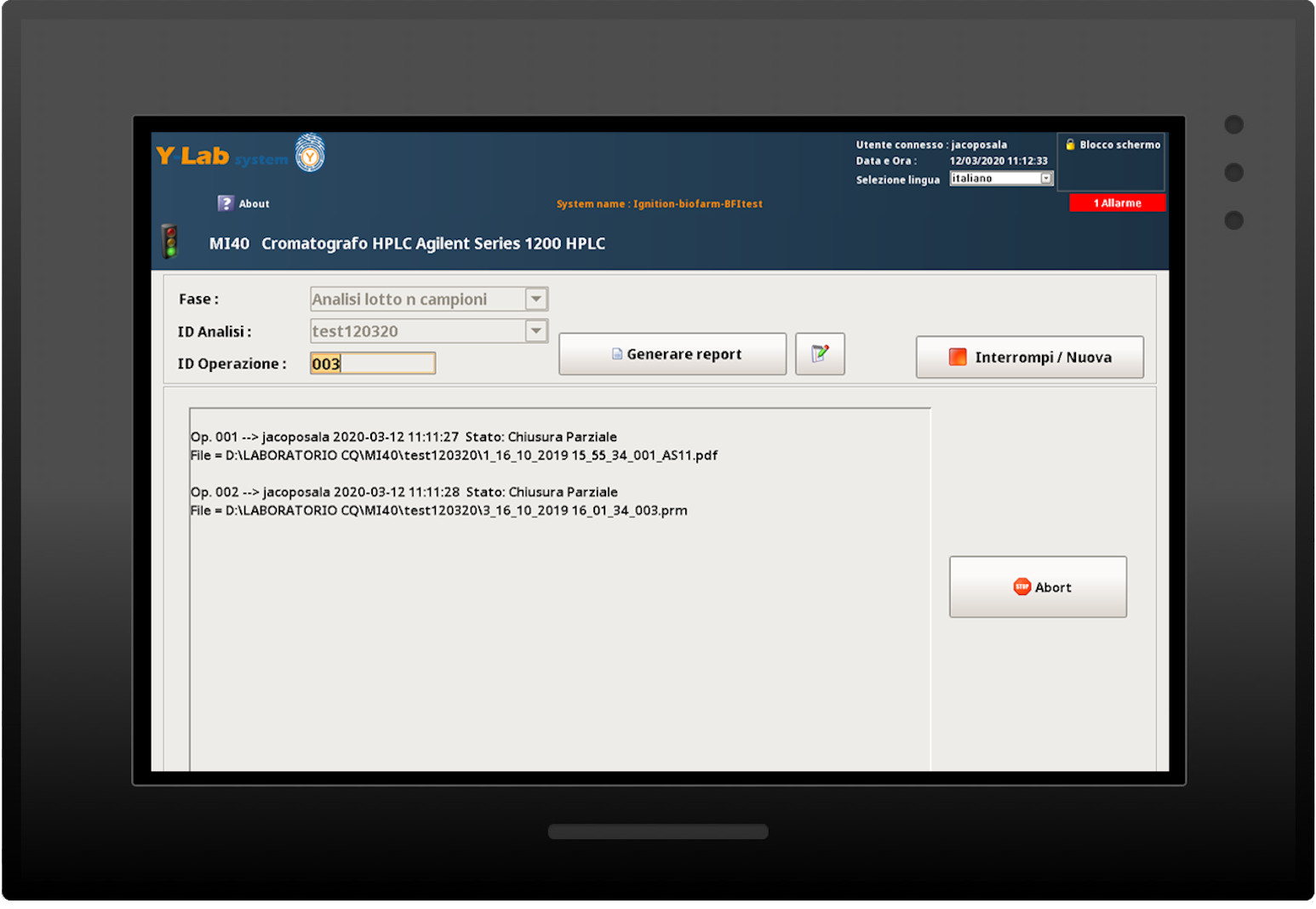
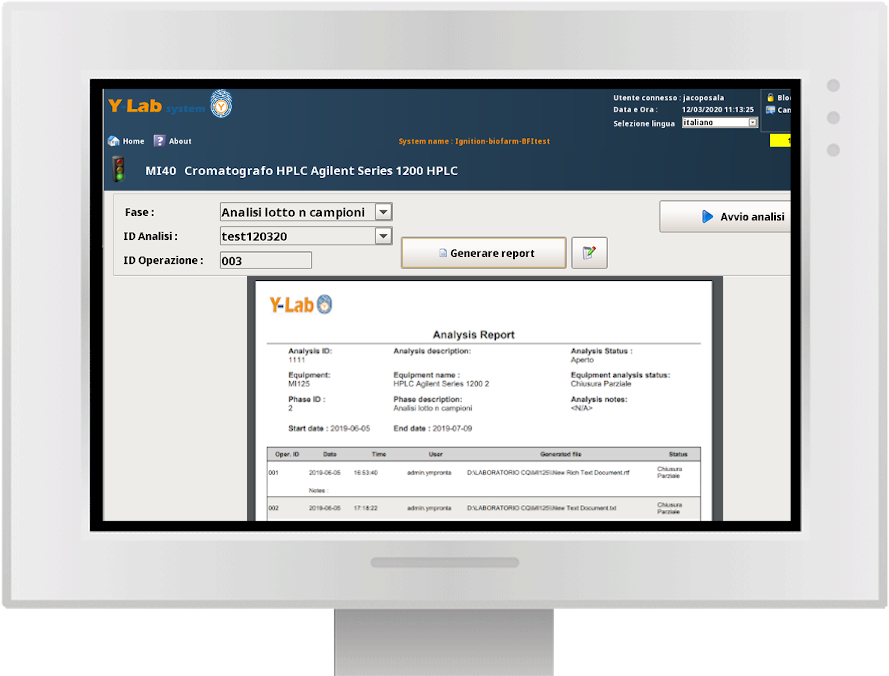
Real-time analytical results and dynamic data
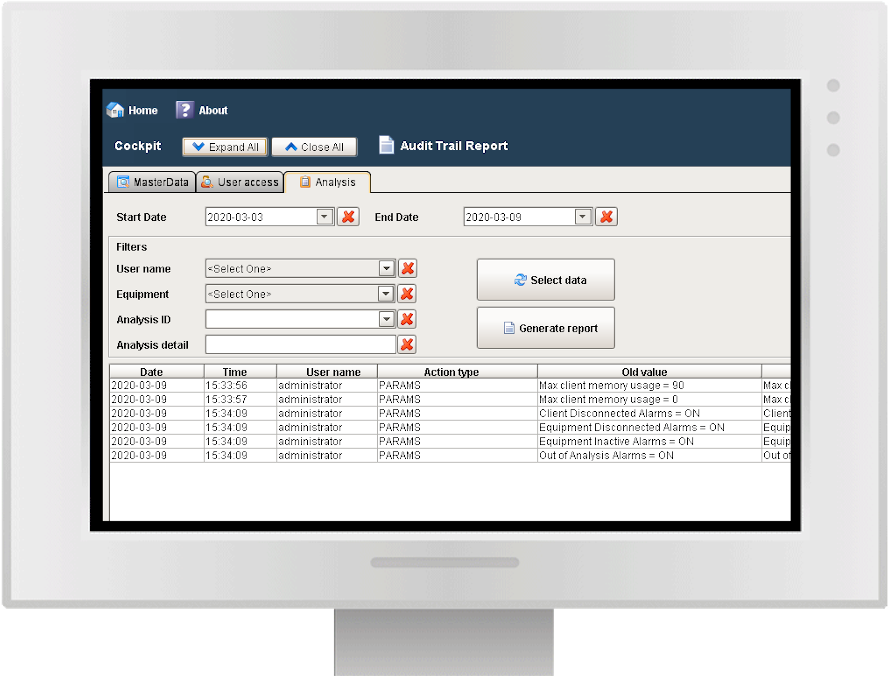
Audit trail and batch report
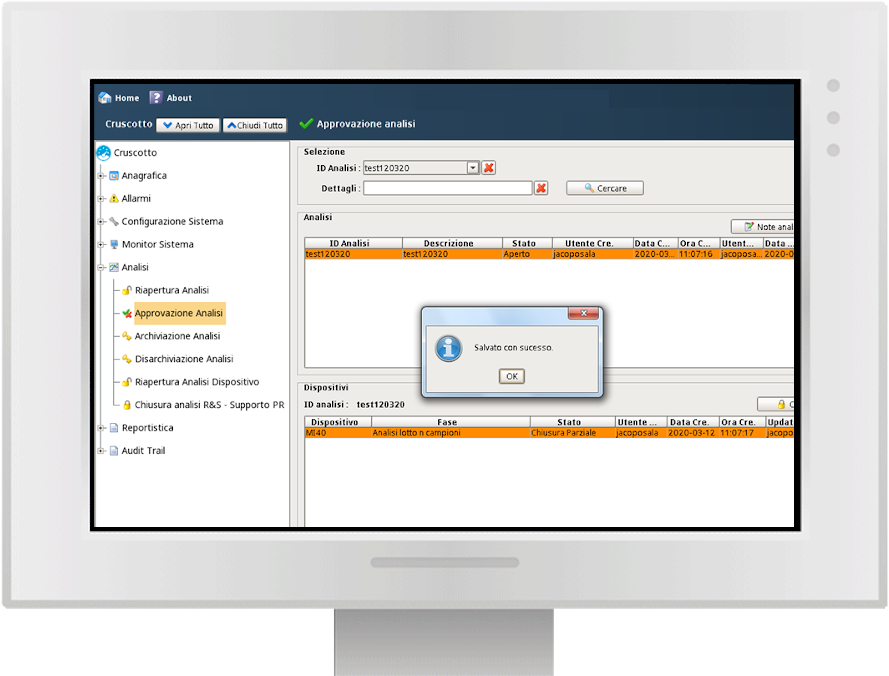
Approval flow
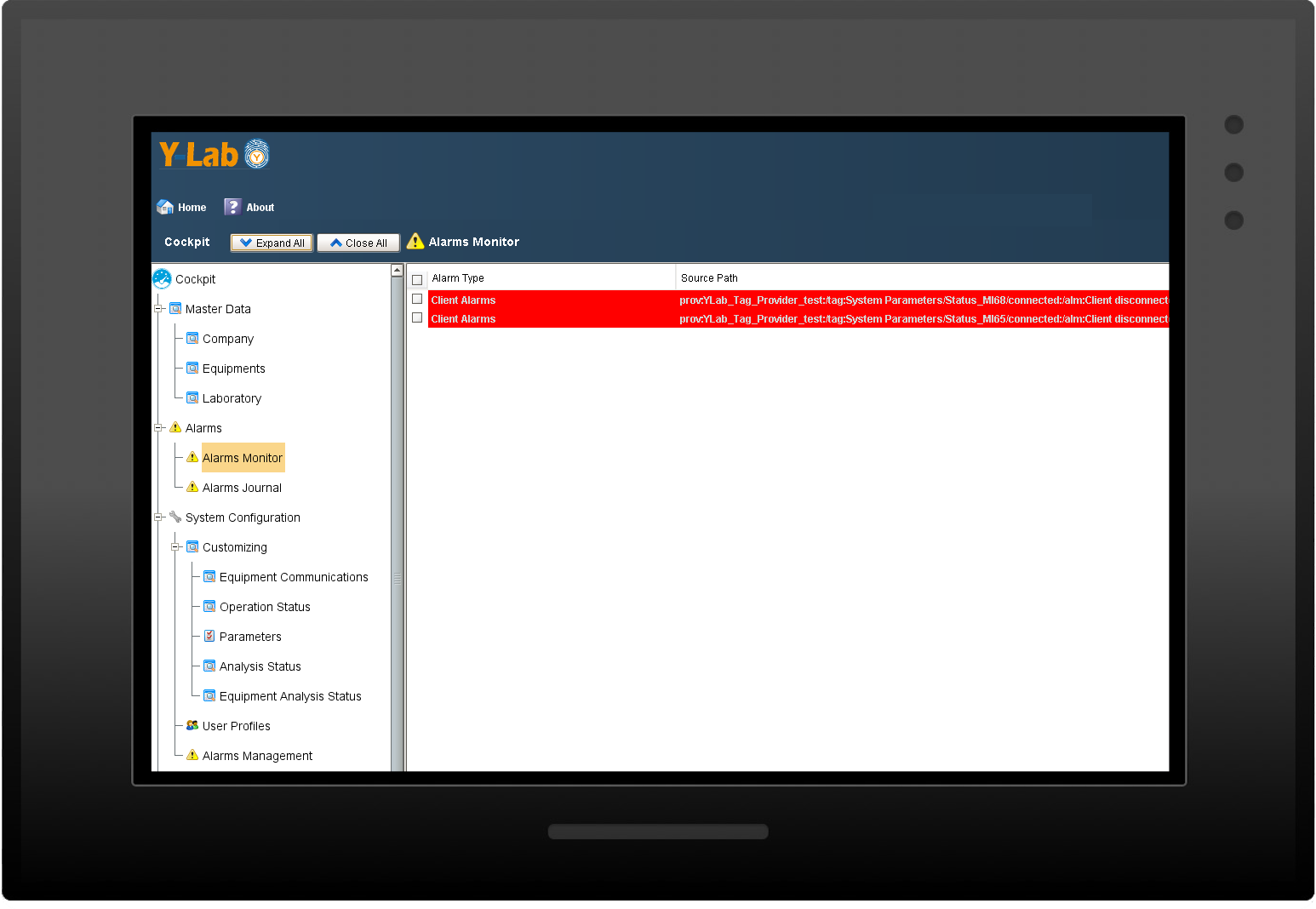
Alarm notifications
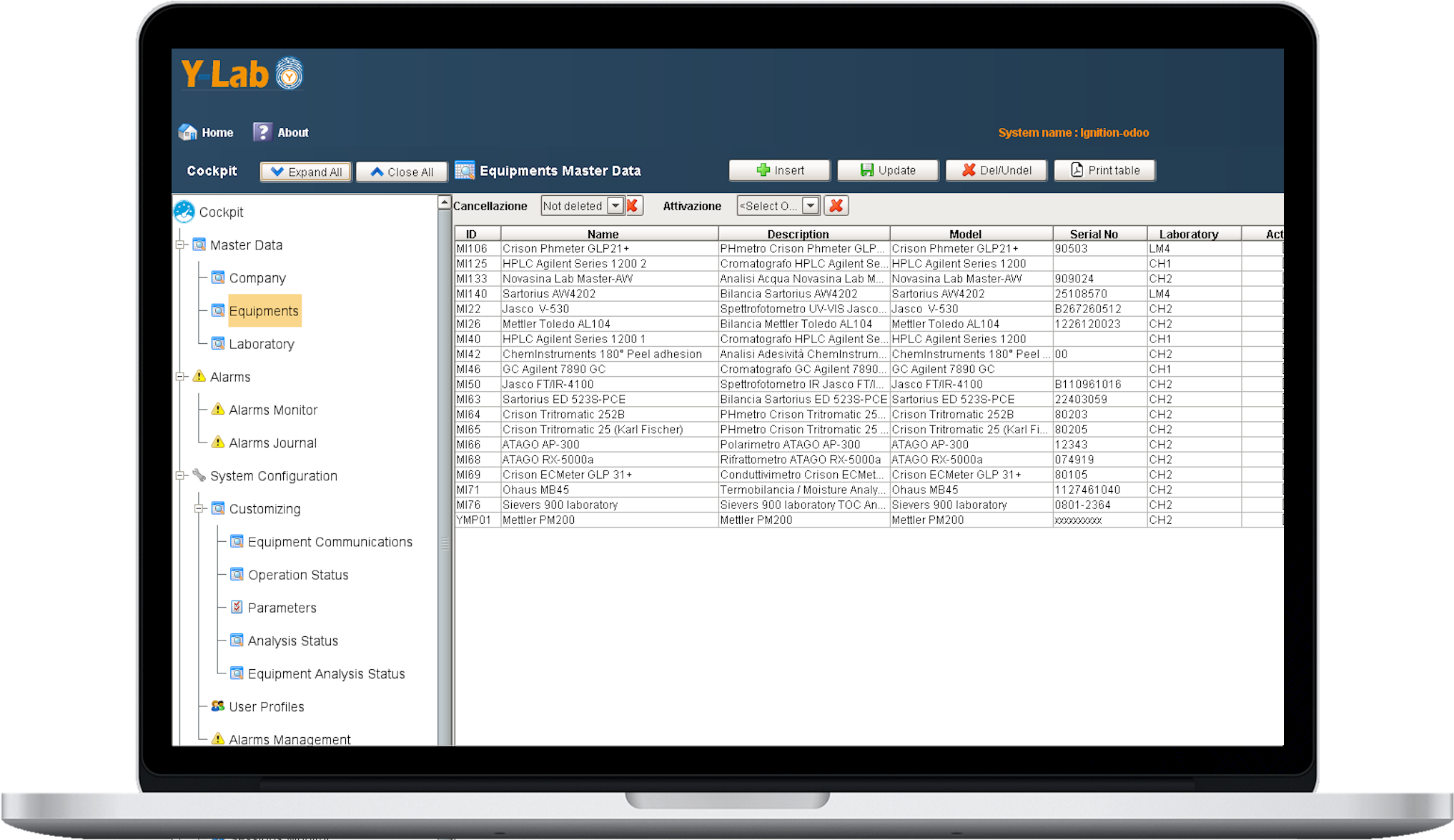
Data governance of master data
Whatever the size of your laboratory, Y-Lab is able to connect with all devices, at no additional license cost.
Data integrity compliance
Gxp master data
real time dynamic data acquisition
Multipurpose devices integration
Audit trail and batch report
approval flow
Classification by laboratory area and type of data acquisition model:
- RT - Real Time Process Data
- SS - Start & Stop Process data
- FT - File transfer
Y-Lab records all the heterogeneous data from the devices (files, values, tags) in real time and historicises them in the DB, making the reporting and Audit Trail accessible (accesses, user / machine actions, changes and registry approvals).
Y-LAB is different
Call us and discover the innovative Y-Lab approach to Data Integrity
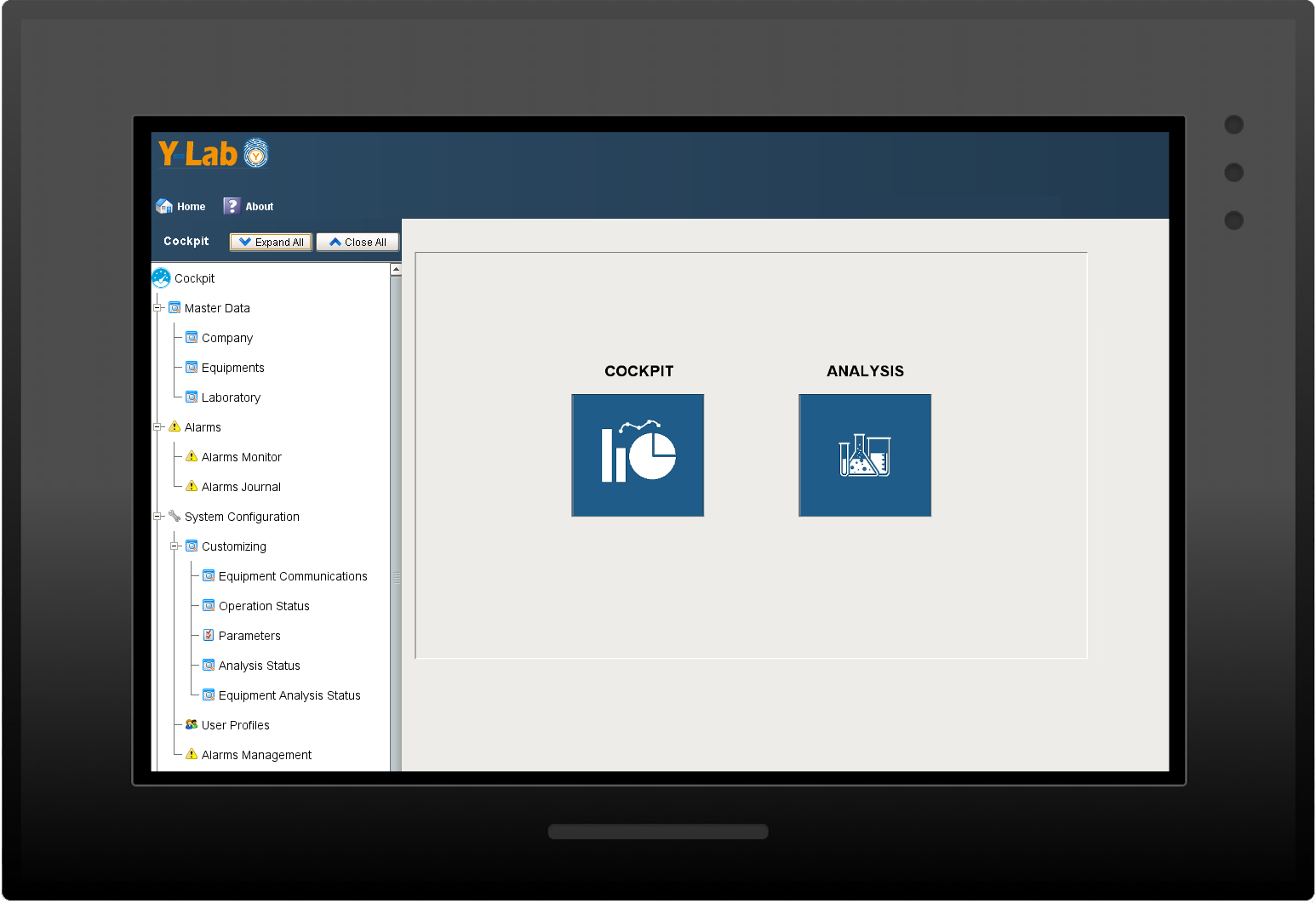
Y-Lab is a level 2-3 application (according to the classification of the industrial standard ISA-95) that allows you to manage the entire fleet of quality control laboratory devices,
operating under the Data Integrity for each of them
.
With this in mind, the client-server architecture has been adopted, i.e. the management logic is performed on the central server
which remotely controls all devices (clients). This choice is dictated by the need to meet the regulations on Data Integrity which, among the various specific requirements, includes that of storing the data generated by each device in a secure archive. In order not to have to replicate this practice individually on each device, the most logical choice is to adopt a centralized architecture
.
Y-Lab exploits this need to its advantage and, if installed on the customer's server, adopts the model of unlimited licenses (limitlless), based on the modules installed on the server and not on the number of devices or number of users. As a result, the license cost calculated per device decreases as the number of devices increases.
Another alternative is to adopt the Y-Lab as-a-service solution (on the Ympronta Cloud platform) which involves several pros and cons:
-
provides an advantageous annual (or monthly) usage fee
-
it does not require the installation of the local server at the customer
-
requires a stable internet connection
-
provides for a minimum contractual term (to be defined)
Operating under Data Integrity is not the only benefit. Y-Lab is a real management software for the laboratory. It can be used as an add-on module to the LIMS. Therefore, in addition to offering compliance with current regulations, the price includes the optimization of company flows and the elimination of paper.
Furthermore, Y-LAB is versatile to the point of having different fields of application (production, process controls, warehouse, advanced reporting) without additional costs in terms of licenses.