eQMS
The first and only Cloud-native Life Sciences quality management system
Start with the basic features and add all the others whenever you want.
The company's business is growing but not the fee.
Quality made simple
A cloud-based quality management system that helps Life Science companies manage all aspects of quality
Full 21 CFR Part 11 and Annex 11 compliant
Our holistic approach allows you to manage the entire quality cycle efficiently and effectively and in full compliance with regulations.
No hidden fees
All features are included in the subscription, without hidden costs, and without frequent interventions. It allows you to be operational in a very short time with a significantly lower cost than in the past.
Constant support along the way
Expert guidance always on hand to ensure the system continues to support your needs as your business grows.
It grows together with the company
Start with the essential, without stressing the entire company by maintaining quality processes, adding new ones and integrating them with other systems, and easily resize everything as needed.
Unique implementation model
Unique Implementation model but customized? Yup! Thanks to the power of technology. Up and running in 6-8 weeks with a fully validated system tailored to your needs.
The best features you can desire
Pre-configured solutions to start off on the right foot. And over time we adapt the system to your needs... at NO extra fees!
Digitizing your quality system has never been easier and the results will take your business to the next level.
controlled documents
A single digital vault, compliant with regulations, for all controlled documents (SOP, policies, work instructions, etc)
Training management
Staff always aligned to their duties thanks to timely training and an advanced monitoring system.
complaints
Customers enabled to register complaints and to follow the entire path of investigation of the causes and improvement over time.
change control
Advanced Change Management thanks to the documentation, approval, planning, and implementation of Change Requests.
QUALITY EVENTS
Facilitated recording of deviations and out-of-specs, connection with any impacted objects within the company, flexibility, and complete visibility of events to anticipate possible consequences.
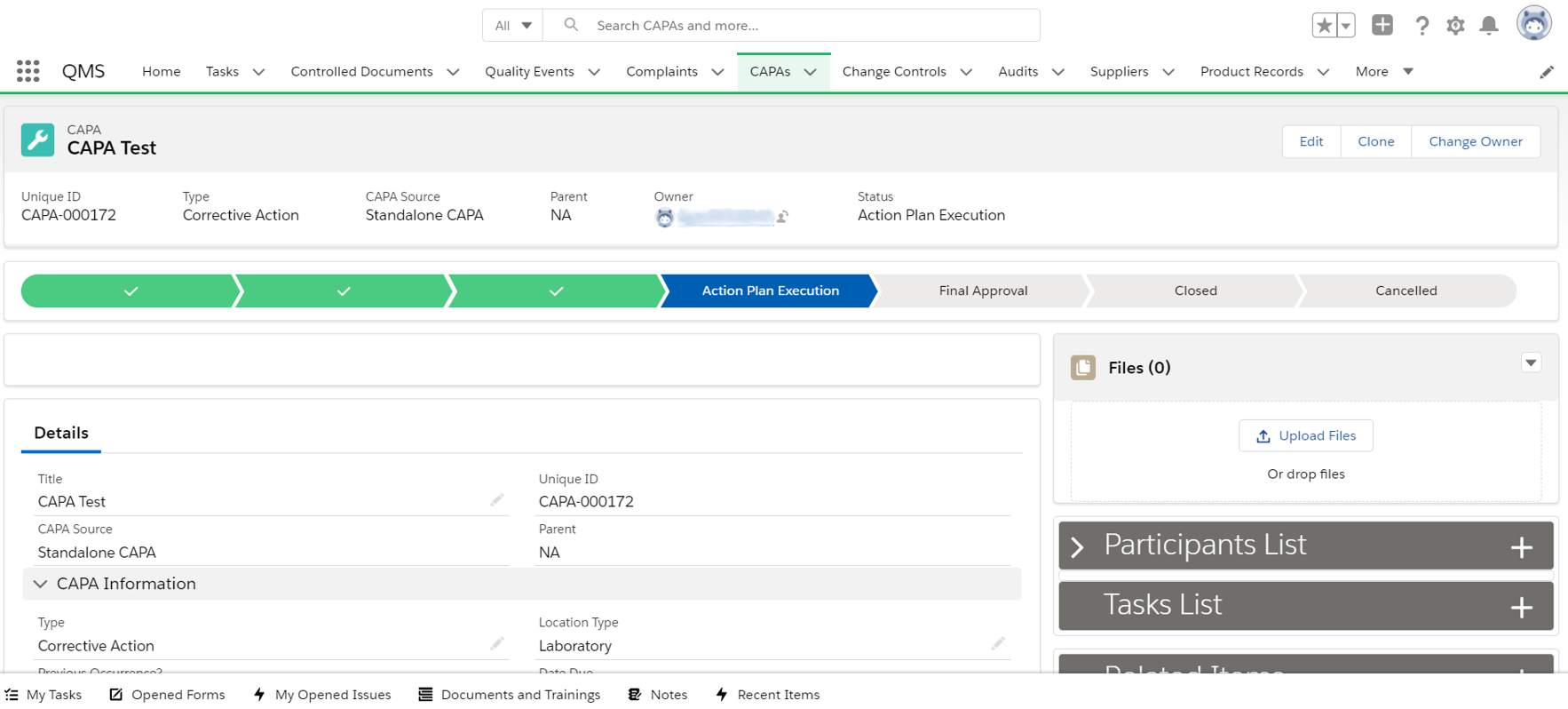
capa
Defining corrective and preventive actions by linking them to any record, event, complaint or inspection allows the company to introduce timely solutions and avoid recurrences.
AUDITs & INSPECTIONS
Efficient management of external, internal, or supplier inspections, recording, and monitoring of observations thanks to their conversion into CAPA, connection with any impacted object or area.
supplier management
Centralized management of all partners, contract manufacturers, suppliers of machinery, software solutions or services. Transparent and effective qualification and collaboration processes.
batch release
The centralized QMS integrated with all business systems and processes is the ideal solution for the batch approval process. Registration of the results of the checks and approval of the lot by the QP with one click.
ENVIRONMENTAL HEALTH & SAFETY (EHS)
Integrated EHS system for the management of all processes relating to environmental events and safety issue, with extensive documentation required by local and national authorities.
EQUIPMENT & CALIBRATION
Consolidated and centralized management of validated corporate assets, their master data, planning, execution, and documentation of maintenance and calibration activities.
risk management
Any change involves a risk that must be managed. the eQMS has advanced risk management tools like risk catalogs, risk definition for any record, complete risk assessment, global monitoring.
You don't need to choose.
All modules are included by default!